A novel mechanism for overexpression of c-Myc, a proto-oncogene in cancer cells
The deubiquitinating enzyme USP17 regulates c-Myc levels and controls cell proliferation and glycolysis
We identified USP17 as a novel deubiquitinating enzyme regulating c-Myc levels to control cell proliferation. Overexpression of USP17 stabilized the c-Myc protein by promoting its deubiquitination, while USP17 knockdown promoted c-Myc degradation and reduced c-Myc levels. The knockdown of USP17 also suppressed cell proliferation and glycolysis. Collectively, the present results reveal a novel role for USP17 in the regulation of c-Myc stability, and suggest its potential as a therapeutic target for cancer treatment.
Full text of release
The c-Myc oncoprotein is frequently overexpressed in human cancers and is essential for cancer cell proliferation. The dysregulation of ubiquitin-proteasome-mediated degradation is one of the contributing factors to the up-regulated expression of c-Myc in human cancers.
In the present study, Dr. Hidetoshi Hayashi (Professor, Nagoya City University) and collaborators herein identified USP17 as a novel deubiquitinating enzyme that regulates c-Myc levels and controls cell proliferation and glycolysis. The overexpression of USP17 stabilized the c-Myc protein by promoting its deubiquitination. In contrast, the knockdown of USP17 promoted c-Myc degradation and reduced c-Myc levels. The knockdown of USP17 also suppressed cell proliferation and glycolysis. Collectively, the present results reveal a novel role for USP17 in the regulation of c-Myc stability, and suggest its potential as a therapeutic target for cancer treatment.
Reference
DOI:10.1002/1873-3468.14296
Funding information
1. Japan Society for the Promotion of Science(18K06660, 20K07052, 21H02650, 18K16081)
2. Nagoya City University(2021103, 2022005)
3. Japan Science and Technology Agency SPRING(JPMJSP2130)
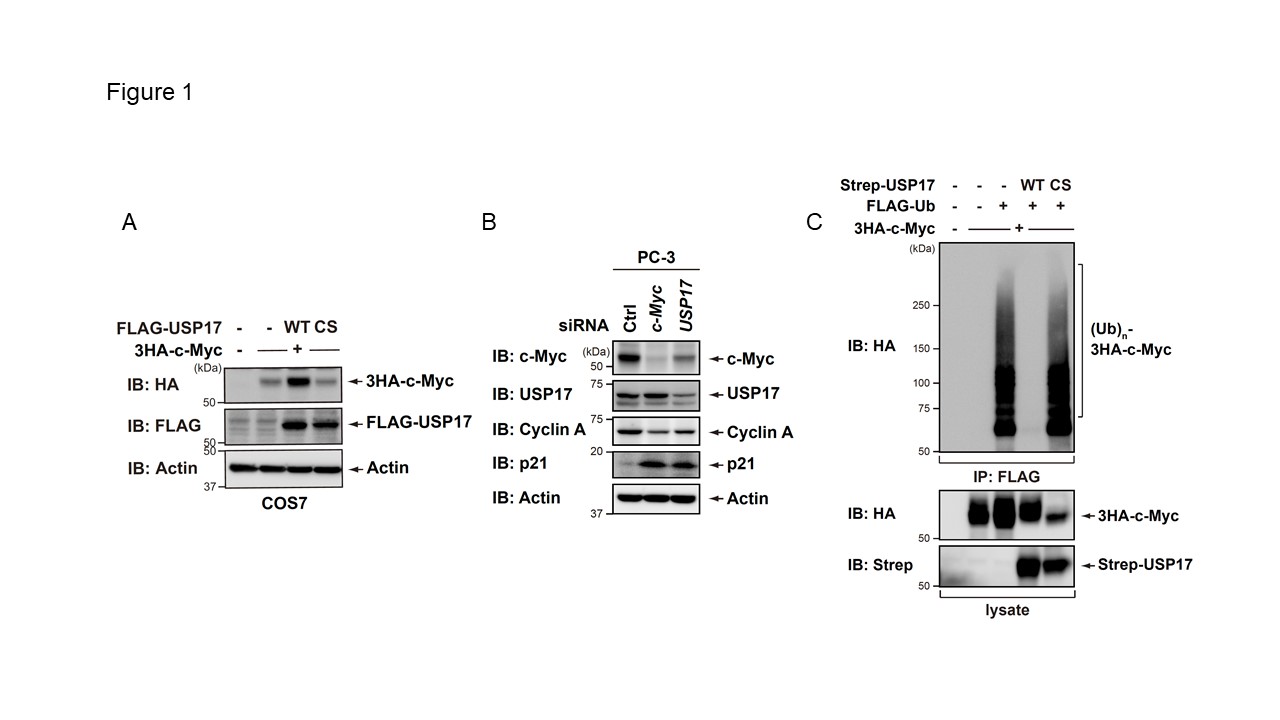
Figure1
USP17 deubiquitinates to stabilize the c-Myc protein.
A. 3HA-c-Myc was co-expressed with FLAG-USP17 (WT or the catalytically inactive mutant (C89S)) in COS7 cells. After 24 h, cell lysates were immunoblotted with the indicated antibodies. CS, C89S. B. PC-3 cells were transiently transfected with the indicated siRNAs. After 72 h, an immunoblot analysis was performed with the indicated antibodies. Ctrl, control. C. An empty vector or plasmid expressing USP17 WT or C89S was co-expressed with 3HA-c-Myc and a FLAG-Ub plasmid into COS7 cells. Cells were treated with 20 microM MG132. After 4 h, cell lysates were analyzed by a pulldown assay and immunoblotting with the indicated antibodies. CS, C89S.
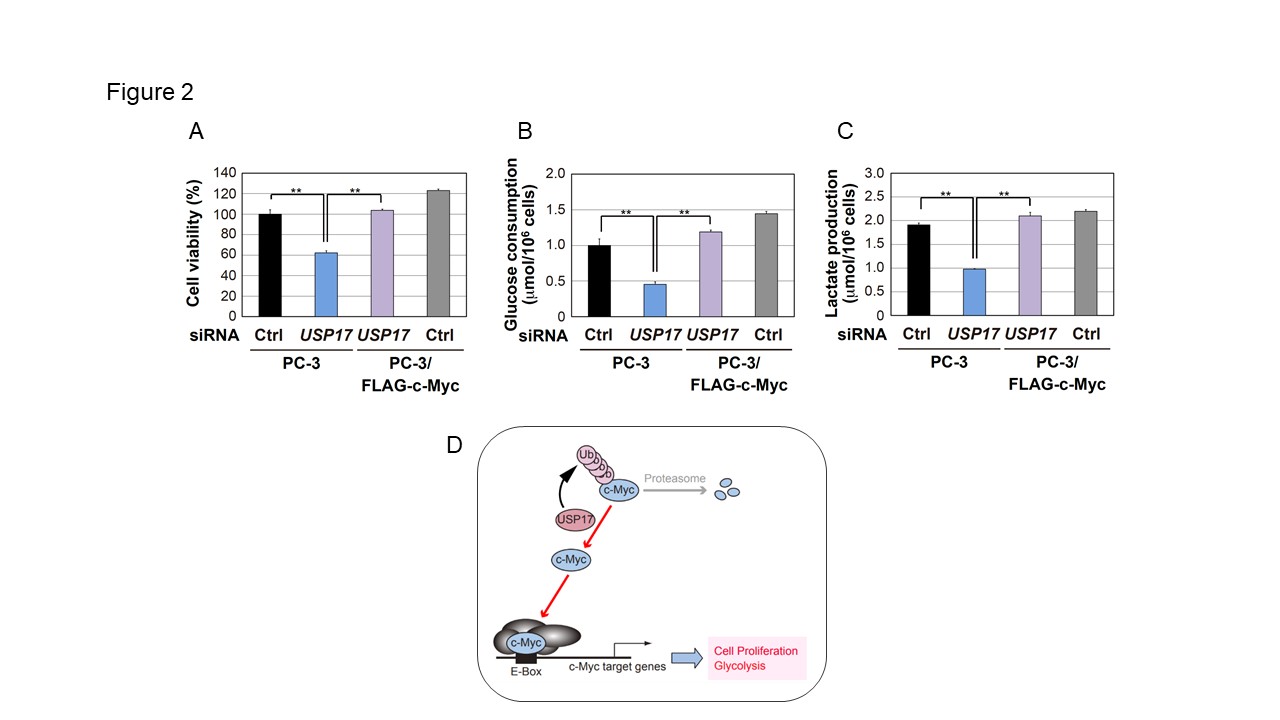
Figure2
USP17 regulates c-Myc-dependent cell proliferation and glycolysis.
A. PC-3 cells and PC-3/FLAG-c-Myc cells were transiently transfected with the indicated siRNAs. After 72 h, cell viability was measured by a WST-8 cell proliferation assay. Results are shown as means ± S.D. (n=3). B, C. The medium from the PC-3 cell culture or PC-3/FLAG-c-Myc cell culture was collected for the analysis of glucose consumption (B) and lactate production (C). Results are shown as means ± S.D. (n=3). D. In cancer cells, a deubiquitinating enzyme, USP17 accumulates c-Myc protein to promote cell proliferation and glycolysis.
| Title of original paper: | The deubiquitinating enzyme USP17 regulates c-Myc levels and controls cell proliferation and glycolysis |
| Journal | FEBS Letters |
| Original Source URL | https://febs.onlinelibrary.wiley.com/doi/full/10.1002/1873-3468.14296 |